|
A chloroplast enzyme safeguards plants against pathological protein aggregation that causes Huntington’s and other neurodegenerative diseases / new research reported in “Nature Aging” may have found a way to “copy” the mechanism for application in human cells
Researchers at the University of Cologne’s CECAD Cluster of Excellence for Aging Research and the CEPLAS Cluster of Excellence for Plant Sciences have found a promising synthetic plant biology approach for the development of a therapy to treat human neurodegenerative diseases, especially Huntington’s disease. In their publication “In-planta expression of human polyQ-expanded huntingtin fragment reveals mechanisms to prevent disease-related protein aggregation” in Nature Aging, they showed that a synthetic enzyme derived from plants – stromal processing peptidase (SPP) – reduces the clumping of proteins responsible for the pathological changes in models of Huntington’s disease in human cells and the nematode Caenorhabditis elegans. Huntington’s disease is among the so called polyglutamine (polyQ) diseases, a group of neurodegenerative disorders caused by multiple repetitions of glutamine amino acids in specific proteins. An excessive number of polyQ repeats can cause proteins to aggregate or accumulate in harmful and damaging protein deposits, leading to cellular dysfunction and death. To date, nine polyQ disorders have been described in humans. They all remain incurable. Among them, Huntington’s disease is an inherited condition that causes widespread deterioration in the brain and disrupts thinking, behavior, emotion and movement. Plants are immune to harmful protein aggregation In their recent study, Professor Dr David Vilchez (CECAD) and Dr Ernesto Llamas (CEPLAS) followed an unconventional approach to find potential drugs to treat polyQ diseases like Huntington’s. Plants are constantly challenged by the environment, but they cannot move to escape from these conditions. However, plants possess a striking resilience to stress that allows them to live long. Unlike humans who suffer from proteinopathies caused by the toxic aggregation or cluster of proteins, plants do not experience these kinds of diseases. They express hundreds of proteins containing polyQ repeats, but no pathologies from these factors have been reported. To explore how plants deal with toxic protein aggregation, Dr Ernesto Llamas, first author of the study, and colleagues introduced the toxic mutant protein huntingtin in plants, which causes cell death in human neurons. In contrast to animal and human models, they found that Arabidopsis thaliana plants actively removed huntingtin protein clumps and avoid harmful effects. By means of synthetic biology, the scientists then transferred the plants’ ability to avoid aggregation into human cultivated cells and animal models of Huntington’s disease. Their hope is that the use of plant proteins could lead to new therapeutic approaches for treating Huntington’s disease and other neurodegenerative diseases. “We were surprised to see plants completely healthy, even though they were genetically producing the toxic human protein. The expression of mutant huntingtin in other models of research like human cultured cells, mice and nematode worms induce detrimental effects and symptoms of disease,” said David Vilchez. Plant protein alleviates symptoms in human cells and nematodes The next step was to discover how plants avoided the toxic aggregation of mutant huntingtin. Indeed, the scientists discovered that the chloroplasts, the plant-specific organelles that perform photosynthesis, were the reason why plants do not show toxic protein deposits. Llamas said: “Unlike humans, plants have chloroplasts, an extra cellular type of organelle that could provide an expanded molecular machinery to get rid of toxic protein aggregates.” The multidisciplinary team identified the chloroplast plant protein SPP as the reason why plants are unaffected by the problematic human protein. Producing the plant SPP in models of Huntington’s disease such as human cultured cells and worms like the nematode C. elegans reduced protein clumps and symptoms of disease. “We were pleased to observe that expression of the plant SPP protein improved motility of C. elegans worms affected by huntingtin even at later aging stages where the symptoms are even worse,” said Dr Hyun Ju Lee, a postdoc also involved in the study. The results thus open the door to testing SPP as a potential therapy for Huntington’s disease. Plants as models for aging research Llamas is convinced that plant research can make a meaningful contribution to treating human diseases. “Many people don’t notice that plants can persist amongst variable and extreme environmental conditions that cause protein aggregation. I believe that plant molecular mechanisms hold the key to discovering new drugs that can prevent human diseases. We usually forget that some plants can live thousands of years and should be studied as models of aging research.” Dr Seda Koyuncu, another postdoc involved in the study, added: “Over the past years, we have seen several promising approaches to treating hereditary diseases like Huntington’s fail. We are confident that our plant synthetic approach will lead to significant advances in the field.” The team has since acquired funding form the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung – BMBF) through the GO-Bio initial program. “We want to bring our idea into an application. Our plan is to found a start-up to produce plant-derived therapeutic proteins and to test them as potential therapeutics to treat neurodegenerative diseases in humans,” said Llamas. The research was conducted at the University of Cologne’s CECAD Cluster of Excellence in Aging Research and CEPLAS Cluster of Excellence on Plant Sciences.
0 Comments
11/15/2023 0 Comments Cold is beneficial for healthy agingA lower body temperature is one of the most effective mechanisms to prolong the lifespan of animals. Writing in ‘Nature Aging’, a working group at the University of Cologne’s CECAD Cluster of Excellence in Aging Research has now described precisely how this works. The scientists show that cold can prevent the pathological aggregation of proteins typical for two aging-associated neurodegenerative diseases.
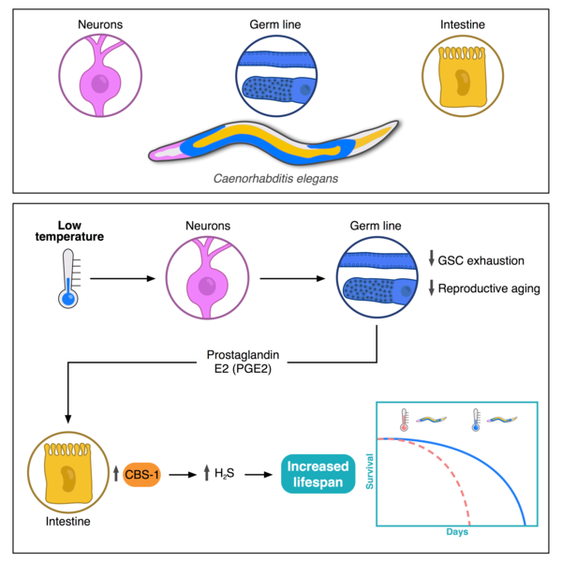
Cold activates a cellular cleansing mechanism that breaks down harmful protein aggregations responsible for various diseases associated with aging. In recent years, studies on different model organisms have already shown that life expectancy increases significantly when body temperature is lowered. However, precisely how this works has still been unclear in many areas. A research team at the University of Cologne’s CECAD Cluster of Excellence in Aging Research has now unlocked one responsible mechanism. The study ‘Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes’ has appeared in Nature Aging. Professor Dr David Vilchez and his working group used a non-vertebrate model organism, the nematode Caenorhabditis elegans, and cultivated human cells. Both carried the genes for two neurodegenerative diseases which typically occur in old age: amyotrophic lateral sclerosis (ALS) and Huntington’s disease. Both diseases are characterized by accumulations of harmful and damaging protein deposits – so-called pathological protein aggregations. In both model organisms, cold actively removed the protein clumps, thus preventing the protein aggregation that is pathological in both ALS and Huntington’s disease. More precisely, the scientists explored the impact of cold on the activity of proteasomes, a cellular mechanism that removes damaged proteins from cells. The research revealed that the proteasome activator PA28γ/PSME3 mitigated the deficits caused by aging in both the nematode and in the human cells. In both cases, it was possible to activate proteasome activity through a moderate decrease in temperature. “Taken together, these results show how over the course of evolution, cold has preserved its influence on proteasome regulation – with therapeutic implications for aging and aging-associated diseases” said Professor Vilchez. Aging is a major risk factor for several neurodegenerative diseases associated with protein aggregation, including Alzheimer’s, Parkinson’s, Huntington’s and ALS. Professor Vilchez said: “We believe that these results may be applied to other age-related neurodegenerative diseases as well as to other animal species.” A key finding was that the proteasome activity can also be increased by genetic overexpression of the activator. That way, disease-causing proteins can be eliminated even at the normal body temperature of 37 degrees Celsius. These results may provide therapeutic targets for aging and aging-associated diseases. It has long been known that while extremely low temperatures can be harmful to organisms, a moderate reduction in body temperature can have very positive effects. For example, a lower body temperature prolongs the longevity of cold-blooded animals like worms, flies or fish, whose body temperature fluctuates with the temperature of the environment. However, the same phenomenon also applies to mammals, who maintain their body temperature within a narrow range no matter how cold or warm their environment is. For example, the nematode lives much longer if it is moved from the standard temperature of 20 degrees Celsius to a colder temperature of 15 degrees Celsius. And in mice, a slight decrease in body temperature of just 0.5 degrees significantly extends their lifespan. This supports the assumption that temperature reduction plays a central role in longevity in the animal kingdom and is a well-conserved evolutionary mechanism. Even in humans, a correlation between body temperature and lifespan has been reported. Normal human body temperature is between 36.5 and 37 degrees Celsius. While an acute drop in body temperature below 35 degrees leads to hypothermia, human body temperature fluctuates slightly during the day and even reaches a cool 36 degrees during sleep. Interestingly, a previous study reported that human body temperature has steadily declined by 0.03 degrees Celsius per decade since the Industrial Revolution, suggesting a possible link to the progressive increase in human life expectancy over the last 160 years. The research was conducted at the University of Cologne’s CECAD Cluster of Excellence in Aging Research. 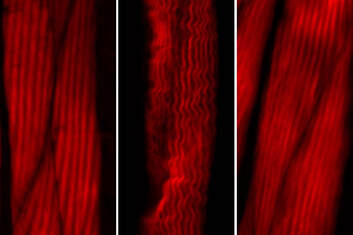 Left: Muscle actin cytoskeleton in young animals. Middle: Muscle actin cytoskeleton in old animals with destabilization of muscle cytoskeleton due to aging Right: Prevention of destabilization of muscle cytoskeleton in old animals by lowering the age-dysregulated high levels of EPS-8, a regulator of actin cytoskeleton. ©Vilchez Lab Left: Muscle actin cytoskeleton in young animals. Middle: Muscle actin cytoskeleton in old animals with destabilization of muscle cytoskeleton due to aging Right: Prevention of destabilization of muscle cytoskeleton in old animals by lowering the age-dysregulated high levels of EPS-8, a regulator of actin cytoskeleton. ©Vilchez Lab he attachment of the small protein ubiquitin to other proteins (ubiquitination) regulates numerous biological processes, including signal transduction and metabolism / Scientists at the University of Cologne discover the link to aging and longevity / Publication in 'Nature'. Scientists have discovered that the protein ubiquitin plays an important role in the regulation of the aging process. Ubiquitin was previously known to control numerous processes, such as signal transduction and metabolism. Prof. Dr. David Vilchez and his colleagues at the CECAD Cluster of Excellence for Aging Research at the University of Cologne performed a comprehensive quantitative analysis of ubiquitin signatures during aging in the model organism Caenorhabditis elegans, a nematode worm which is broadly used for aging research. This method - called ubiquitin proteomics - measures all changes in ubiquitination of proteins in the cell. The resulting data provide site-specific information and define quantitative changes in ubiquitin changes across all proteins in a cell during aging. A comparison with the total protein content of a cell (proteome) showed which changes have functional consequences in protein turnover and actual protein content during aging. The scientists thus discovered new regulators of lifespan and provide a comprehensive data set that helps to understand aging and longevity. The article, 'Rewiring of the ubiquitinated proteome determines aging in C. elegans,' has now been published in Nature. ‘Our study of ubiquitin changes led us to a number of exciting conclusions with important insights for understanding the aging process," said Dr Seda Koyuncu, lead author of the study. "We discovered that aging leads to changes in the ubiquitination of thousands of proteins in the cell, whereas longevity measures such as reduced food intake and reduced insulin signaling prevent these changes.’ Specifically, the researchers found that aging causes a general loss of ubiquitination. This is caused by the enzymes that remove ubiquitin from proteins become more active during aging. Normally, ubiquitinated proteins are recognized and destroyed by the proteasome, the cell's garbage truck. The scientists showed that the longevity of organisms is determined by age-related changes in the degradation of structural and regulatory proteins by the proteasome. "We studied animals with a defective proteasome to identify proteins that become less ubiquitinated with age and thus are not cleaned up by the proteasome and accumulate in the cell. The resulting protein accumulation leads to cell death," Koyuncu says. "Remarkably, we saw that reducing the protein levels of these untagged proteins was sufficient to prolong longevity, while preventing their degradation by the proteasome shortened lifespan." In addition to providing a comprehensive data set, the investigators showed that defining changes in the ubiquitin-modified proteome can lead to the discovery of new regulators of lifespan and aging traits. They focused their follow-up analyses on two specific proteins that lacked ubiquitin labeling during aging. IFB-2, a protein important for cell structure, and EPS-8, a modulator of a signaling pathway that regulates a variety of cellular processes. These proteins, which are no longer adequately labeled in aged organisms, affect longevity in a variety of tissues. Increased protein levels of IFB-2, for example, cause the intestine to fail to digest properly or absorb nutrients and also make it more susceptible to bacterial infections, which is a characteristic of aging animals. "Remarkably, knockdown of IFB-2 in adult C. elegans was enough to restore normal gut function," Koyuncu says. Too much amounts of EPS-8 in cells over activate a specific signaling pathway (RAC) in muscle and brain cells. The team discovered here that the RAC signaling pathway determines longevity, muscle integrity and motility. "Our findings may point to new ways to delay the aging process and improve quality of life in old age. In particular, we have established a novel link between aging and general changes in the ubiquitin-modified proteome, a process that actively influences longevity," said study coordinator David Vilchez, research group leader at CECAD and the Center for Molecular Medicine Cologne (CMMC). "Our results and rich datasets may have important implications for several research priorities, including aging, ubiquitination and other cellular processes." Original publication: Koyuncu S, Loureiro R, Lee HJ, Wagle P, Krueger M, Vilchez D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 2021 https://www.nature.com/articles/s41586-021-03781-z 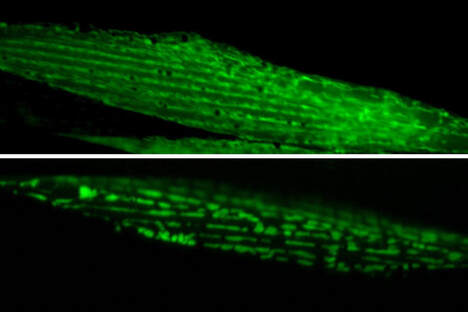 On top: mitochondria in healthy muscle of C. elegans. Bottom: fragmented mitochondria in the muscle after induction of aggregation of proteins in the germline. Picture: Guiseppe Calculli On top: mitochondria in healthy muscle of C. elegans. Bottom: fragmented mitochondria in the muscle after induction of aggregation of proteins in the germline. Picture: Guiseppe Calculli Scientists from the University of Cologne found that the balance status of proteins (protein homeostasis) of germline cells influences protein aggregation in other tissues by long-distance signaling | Publication in ‘Science Advances’ A recent study shows that a healthy reproductive system can prevent disease-related protein accumulation in distant tissues, such as neurons, and alteration of mitochondria - the power plants of cells. An imbalance of proteins, for example an aggregation of damaged proteins in brain cells, can lead to diseases like Alzheimer's, Huntington’s disease or Amyotrophic Lateral Sclerosis (ALS). Since these diseases are associated with aging and the reproductive system is one of the first tissues to decline during aging, Dr. David Vilchez and his group investigated whether the protein homeostasis (proteostasis) of germline cells (the cells that form the egg, sperm and the fertilized egg) influences other tissues and organs. Using the model organism Caenorhabditis elegans, the scientists show that when the germline accumulates damaged protein aggregates, it releases specific signals (Wnt singaling) which in turn induce changes in mitochondria, the powerhouses of the cells, leading to protein aggregation in other tissues such as muscle or neurons. The article ‘Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans’ has now been published in Science Advances. "We were very excited when we saw that just by inducing accumulation of protein aggregates in the germline, we could change the mitochondrial network of the entire organism inducing protein aggregation in neurons," said Guiseppe Calculli, lead author of the study. In the future, the group plans to examine whether germ-line specific proteins also aggregate during the aging process and whether this process contributes to the age-associated aggregation of proteins characteristic of pathologies like Huntington’s disease or ALS. "Our findings open a new door to understanding why protein aggregates accumulate in the neurons of patients with Huntington’s disease and ALS. Since these aggregates can contribute to the neurodegeneration characteristic of these diseases, which remain incurable, a better understanding of the process discovered here might lead to novel therapeutic approaches," explained Vilchez, research group leader at the CECAD Cluster of Excellence in Aging Research and the Center for Molecular Medicine Cologne (CMMC) and leader of this study. Original publication: G. Calculli, H.J. Lee, K. Shen, U. Pham, M. Herholz, A. Trifunovic, A. Dillin and D. Vilchez (2021). Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans. Science Advances 7 (26): eabg3012. https://advances.sciencemag.org/content/7/26/eabg3012 1/20/2021 0 Comments The intrinsic chaperone network of Arabidopsis stem cells confers protection against proteotoxic stress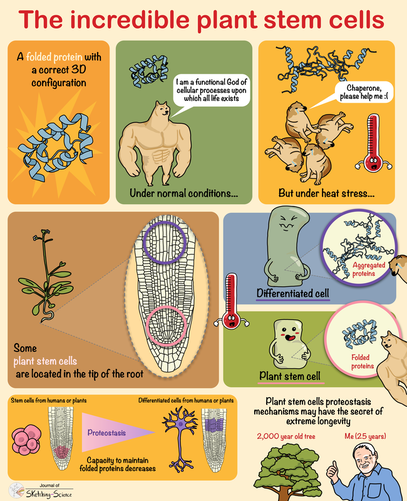 New preprint from the lab! https://www.biorxiv.org/content/10.1101/2021.01.19.427268v1 Here we sought to understand the proteostasis network of plant stem cells and its impact on plant survival under extreme environmental conditions such as high temperature. The comic by Ernesto Llamas summarizes our results. This project was our first “adventure” on plant biology and it was so much fun that we will continue working on that. The study was led by our very talented postdoc Ernesto Llamas. Also, congrats to all the team! Very thankful to our collaborators Alga Zuccaro and Manuel Rodríguez-Concepción and the funding from the ERC and the Humboldt Foundation. Plant stem cells provide new pools of differentiated cells that form organs and rejuvenate or replace damaged tissues. Protein homeostasis (proteostasis) is required for cell function and viability. However, the link between proteostasis and plant stem cells remains unknown. In contrast to their differentiated counterparts, we find that root stem cells can prevent the accumulation of aggregated proteins even under proteotoxic stress conditions such as elevated temperatures. We also find root stem cells express high levels of distinct chaperones that maintain proteome integrity. By mimicking this proteostasis network in other cells, we could prevent protein aggregation in differentiated cells and confer resistance to heat stress in plants. Taken together, our results indicate that enhanced proteostasis mechanisms in stem cells could be an important requirement for plants to persist under extreme environmental conditions and reach extreme long ages.  Loss of ubiquitin-conjugating enzyme leads to impediment in growth of nerve cells/Link found between cellular machineries of protein degradation and regulation of the epigenetic landscape in human embryonic stem cells A group of scientists from CECAD, the Cluster of Excellence ‘Cellular Stress Responses in Aging-Associated Diseases,’ have found a mechanism by which neurodevelopmental diseases concerning neurons can be explained: The loss of a certain enzyme, UBE2K, impeded the differentiation of stem cells by silencing the expression of genes important for neuronal differentiation and, therefore, the development and generation of neurons. More specifically, UBE2K regulates the levels and activation of histones, key proteins that pack and organize the DNA, regulating the expression of genes. Being part of the epigenetic landscape of the cell, the changes made to the histones are reversible and could provide a chance for future developments of treatments for neurodevelopmental diseases. The study is available in the current issue of Communications Biology. Embryonic stem cells (ESCs) can replicate indefinitely while retaining their potential to differentiate into all other types of cells. Thus, nerve cells (neurons), muscle cells and all the other cells of the body are produced in a developing organism. Errors during this process can lead to congenital diseases. Degrading damaged proteins within the cell is an important factor in this process. Thus, the scientists studied the interaction between the proteasome, the main protagonist in terminating proteins, and the epigenetic landscape. Epigenetic landscapes are the heritable changes to an organism which are not determined by DNA but through changes to the chromatin, which can organize and silence DNA into tighter packages. Azra Fatima from CECAD studied the interactions of the histones in immortal human embryonic stem cells (hESCs) which have a unique chromatin architecture and especially low levels of a certain histone called H3 which has undergone a chemical addition in the form of three methylgroups (H3K9me3). Histones are proteins which are part of the chromatin in cell nuclei. They build up spools around which the DNA winds, shortening it by a ratio of 1:10 millions. They are also responsible for regulating the gene expression by which genes produce proteins in the organism. In addition, they play an important role in the process of cellular differentiation in which a cell, for example an embryonic stem cell, changes into another type of cell with a higher degree specialization. They found that embryonic stem cells exhibit high expression of UBE2K (Ubiquitin-conjugating enzyme E2 K), a ubiquitin-conjugating enzyme. These enzymes are known for being important in the process of degradation of proteins. The loss of the enzyme in embryonic stem cells caused an increase in the levels of H3K9 trimethyltransferase SETDB1, resulting in higher trimethylation of H3K9, leading in turn to a repression of neurogenic genes during the differentiation of the stem cells. As a result, the loss of UBE2K impaired the ability of the stem cells to differentiate into neural progenitors a type of precursor cell that generate neurons and other cells of the nervous system. Besides H3K9 trimethylation, the scientists found that UBE2K binds histone H3 to induce its polyubiquitination and degradation by the 26S proteasome. Notably, ubc-20, the worm orthologue of UBE2K, also regulates both histone H3 levels and H3K9 trimethylation in the germ cells of the model organism C. elegans. ‘Our results indicate that UBE2K crosses evolutionary boundaries to promote histone H3 degradation and reduce H3K9me3 repressive marks in immortal cells like human embryonic stem cells and germline cells”, says Fatima. ‘We found a link between the ubiquitin-proteasome system and epigenetic regulation in immortal stem cells,’ Fatima concluded. ‘It would be also interesting to see if UBE2K regulates the epigenetic state in other cell types like cancer cells.’ David Vilchez, the corresponding author of the manuscript, added: ‘We believe that our findings can have important implications to understand the development of the human brain.’ By precisely regulating the levels of UBE2K it would be possible to determine the cell type specific epigenetic landscapes. Different diseases like Huntington’s disease are associated with alterations in epigenetic marks. Since the epigenetic marks are reversible, it will be interesting to study if the epigenetic status of pluripotent stem cells from patients can be modulated by controlling the proteasome system and UBE2K. In order to correct the disease phenotype, novel strategies could be designed to correct epigenetic alterations in early developmental stages and thus provide a potential treatment for diseases. Media Contact: Dr. Azra Fatima [email protected] Press and Communications Team: Robert Hahn [email protected] +49 221 470 2396 Publication: www.nature.com/articles/s42003-020-0984-3 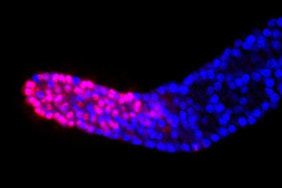 Cold temperature delays the aging of the germline and extends fertility of C. elegans. The germline promotes the fitness of other tissues to induce longevity, as published in ‘Nature Metabolism’. To delay the aging process of the entire organism at cold temperature, germ stem cells communicate with the rest of the body using the hormone prostaglandin, as David Vilchez and his team discovered. A moderate reduction of body temperature can induce a remarkable increase in lifespan. This phenomenon was first described in invertebrates such as worms, and later observed in fish and mice. Although the longevity effects of cold temperature were originally reported over a century ago, little is known about this process. ‘Neurons detect and process cold temperature to delay aging of the reproductive system and therefore extend fertility’, David Vilchez from the Cluster of Excellence CECAD reports. Their new research findings were published in Nature Metabolism. The most important goal of the organism is to proliferate and keep the next generation coming. To assure this, the germline is especially protected. Living beings distribute most of their energetic resources to protect the germline, while less energy goes into protection of muscles, neurons, intestine and other somatic tissues. Therefore, when the germline is removed, organisms tend to live much longer as shown in worms and fruit flies. In these lines, distinct pathways that extend lifespan eventually affect fertility. In their study, the researchers were able to show for the first time that under certain conditions such as cold temperature both goals – longevity and fertility – can be reached without sacrificing one of them. Specific neurons sense low temperature and communicate with the germ stem cells that rejuvenate the reproductive system, David Vilchez explains: “They say: don´t age, everything is alright, you can continue generating new germ stem cells.” Then, germ stem cells release the hormone prostaglandin E2 to maintain the quality of proteins in somatic tissues. In addition, prostaglandin E2 increases the levels of a protein called cbs-1 in the intestine. This protein produces the gas hydrogen sulphide – a substance which is known to prevent neurodegeneration and extend longevity in numerous organisms, including mammals. “High levels of this substance could kill you – but certain physiological levels make the worms and other organisms live longer,” as stated by Hyun Ju Lee and Alireza Noormohammadi, researchers involved in this study. Some mechanisms that extend lifespan lead to miserable-looking worms, laying less eggs and moving hardly. “That wasn´t the case here. Our worms lived long and prosper,” David Vilchez adds. Importantly, the researchers found that increasing hydrogen sulphide in the intestine alone was sufficient to extend lifespan even at high temperatures. The new findings put aging in perspective, as Vilchez says: “Aging is not just a random process, where DNA damages, toxic proteins or other threats accumulate, but rather a regulated process.” Whereas current theories of aging and extensive evidence support that the reproductive system actively promotes the aging of other tissues, the findings indicate that this is not always the case. The researchers observed that increased maintenance of the germline is required for long lifespan at cold temperature. “Our findings provides a proof of regulatory systems that promote both germline and somatic fitness, without the need to sacrifice either fertility or lifespan,” David Vilchez summarizes. Original publication: Prostaglandin signals from adult germ stem cells delay somatic aging of C. elegans Hyun Ju Lee, Alireza Noormohammadi, Seda Koyuncu, Giuseppe Calculli, Milos Simic, Marija Herholz, Aleksandra Trifunovic, David Vilchez Nature Metabolism, DOI: https://doi.org/10.1038/s42255-019-0097-9 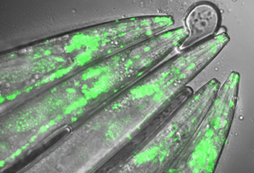 Mechanism that could reduce the toxic aggregation of huntingtin protein discovered / new therapies possible in the long run The neuroscientist Dr David Vilchez and his team at CECAD, the University of Cologne’s Cluster of Excellence for Aging Research, have made an important step towards understanding the mechanisms that cause the neurodegenerative disorder Huntington’s disease. Particularly, they identified a system blocking the accumulation of toxin protein aggregates, which are responsible for neurodegeneration. The results have now been published in the journal ‘Nature Communications’. Huntington’s disease is a neurodegenerative disorder that results in the death of brain cells, leading to uncontrolled body movement, loss of speech and psychosis. Mutations in the huntingtin gene cause the disease, resulting in the toxic aggregation of the huntingtin protein. The accumulation of these aggregates causes neurodegeneration and usually leads to the patient’s death within twenty years after the onset of the disease. To examine the mechanisms underlying Huntington’s disease, Vilchez and his team used so-called induced pluripotent stem cells (iPSC) from Huntington’s disease patients, which are able to differentiate into any cell type, such as neurons. Induced pluripotent stem cells derived from patients with Huntington’s disease exhibit a striking ability to avoid the accumulation of toxic protein aggregates, a hallmark of the disease. Even though iPSCs express the mutant gene responsible for Huntington’s disease, no aggregates were found. The researchers identified a protein called UBR5 as a protective mechanism for the cells, promoting the degradation of mutant huntingtin. These findings can contribute to a better understanding of Huntington’s disease and could be a step stone to developing further treatment in patients. The researchers screened immortal iPSCs from patients and derived neurons for differences in their ability to avoid mutant huntingtin aggregation. They found that huntingtin can be degraded by the cellular disposal system known as the proteasome. However, this system is defective in the neurons, which leads to the aberrant aggregation of the mutant huntingtin protein. Vilchez and his team found that UBR5 is increased in pluripotent stem cells to accelerate the degradation of huntingtin in the cells. To examine the role of UBR5 in the regulation of the mutant huntingtin gene (HTT), they reduced the levels of UBR5 and could immediately see an accumulation of aggregated proteins in iPSCs. ‘This was striking to see’, says Vilchez. ‘From nothing, the cells went to huge amounts of aggregates.’ The authors went a step further and examined whether UBR5 also controls mutant huntingtin aggregation in Huntington’s disease organismal models. Indeed, they found that dysregulation of UBR5 results in a massive increase in the aggregation and neurotoxic effects in neurons. On the other hand, promoting UBR5 activity blocks mutant huntingtin aggregation in the Huntington’s disease models. To test the specificity of the results, the researchers also kept an eye on other illnesses. ‘We also checked the mechanism in other neurodegenerative diseases like amyotrophic lateral sclerosis’, says Seda Koyuncu, a doctoral student working in Vilchez’s lab and a main author of the publication. ‘Our result is very specific to Huntington’s disease’, adds Dr Isabel Saez, another main author working with Vilchez at CECAD. Even though the results could be important for treatment and drug development, there is no therapy yet. ‘It’s not like you discover something new and then there is a cure, it’s more difficult – but in some years there might be a therapy’, Saez comments. Until then, more research needs to be done. CECAD is the Cluster of Excellence at the University of Cologne and Cologne University Hospital conducting research on diseases associated with aging. Its six research areas cover a wide range of basic research on the causes and mechanisms of these diseases. David Vilchez is a Principal Investigator in Research Area B: ‘Disruptions in Protein Metabolism Cause Aging-Associated Diseases’. His research mainly focuses on protein homeostasis regulation in stem cells and aging. Publication: ‘The ubiquitin ligase UBR5 suppresses proteostasis collapse in pluripotent stem cells from Huntington’s disease patients’. Seda Koyuncu, Isabel Saez, Hyun Ju Lee, Ricardo Gutierrez-Garcia, Wojciech Pokrzywa, Azra Fatima, Thorsten Hoppe and David Vilchez. Nature Communications, volume 9, 23 July 2018, article number: 2886 (2018) 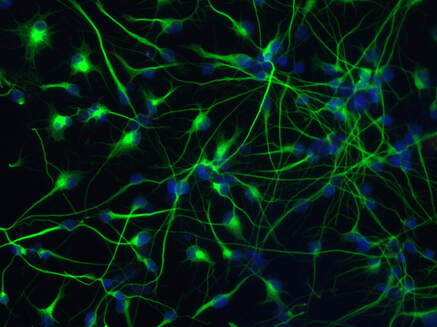 Since their discovery in 2006, induced pluripotent stem cells are a glimmer of hope for many diseases. But further research of the complex regulation of pluripotent stem cell identity revealed unexpected difficulties. A team of researchers at the Cluster of Excellence CECAD has now found an efficient way to produce neurons from pluripotent stem cells. Their research was published in Nature Communications. As the origin of multicellular organisms, pluripotent stem cells can differentiate into all the cell types of the body. These cells can replicate indefinitely in culture and, therefore, are considered immortal. The gold standard of pluripotency is the embryonic stem cell (ESC). Somatic cells such as skin cells can be reprogrammed to generate induced pluripotent stem cells (iPSCs) that share similar characteristics with ESCs. As such, pluripotent stem cells hold a great promise for regenerative medicine as a potential source of healthy differentiated cells, including neurons. Moreover, these cells represent an invaluable resource to investigate human development and disease in the relevant cells (neurons) affected in disorders such as Alzheimer´s, Huntington’s or Parkinson´s. Neuronal differentiation protocols of pluripotent stem cells are usually expensive and generate a mixture of different neuronal cells and other cell types. By knocking down a single gene, the team led by David Vilchez, was able to produce neurons with 100% efficiency: “By silencing one single protein with the gene-editing method CRISPR, the cells spontaneously start to differentiate into neurons! That´s a great and much faster way to increase neurogenesis.” In natural conditions, this factor called CSDE1 prevents differentiation and keeps the cells in a pluripotent state. “This could be a very powerful mechanism to have pure populations of neurons and to facilitate a better understanding of neurodegenerative diseases.” Hyun Ju Lee, first author of the study was most excited about the fast changes observed in her assays: “We could visualize the changes and really see it happening, the differentiation goes really fast. We also double-checked in multiple stem cell lines from different donors and induced pluripotent stem cells and got the same results.” For the study, human embryonic stem cells, induced pluripotent stem cells and mouse stem cells were used. By using the new approach, it would be possible to facilitate the generation of neurons from samples of different patients and study the disease or test pharmaceuticals on it. Even though those results are another step to clinical application, there is still a long way to go, says David Vilchez: “New neurons from the dish could be important for studying diseases like Parkinson´s, Alzheimer´s or Huntington´s, but we are still at the starting point of this exciting research.” The research was a collaboration between CECAD, the Center for Molecular Medicine Cologne and the University Clinic Cologne. Original Publication: A post-transcriptional program coordinated by CSDE1 prevents intrinsic neural differentiation of human embryonic stem cells Hyun Ju Lee, Deniz Bartsch, Cally Xiao, Santiago Guerrero, Gaurav Ahuja, Christina Schindler, James J. Moresco, John R. Yates III, Fátima Gebauer, Hisham Bazzi, Christoph Dieterich, Leo Kurian and David Vilchez Nature Communications, DOI: 10.1038/s41467-017-01744-5 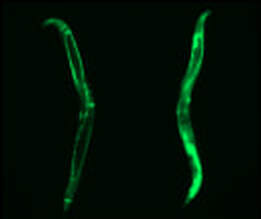 With age, somatic cells such as neurons lose their ability to maintain the quality of their protein content. Pluripotent stem cells, on the contrary, do not age and have increased mechanism to maintain the integrity of their proteins. Researchers from CECAD, the Cluster of Excellence on Aging Research based at the University of Cologne, defined the mechanisms underlying increased protein quality control of pluripotent stem cells. Then, the researchers mimicked these mechanisms in somatic tissues of model organisms to extend lifespan and delay age-related diseases. Their research was published in Nature Communications on November 28. The survival of an organism is linked to its ability to maintain the quality of the cellular proteins. A group of proteins called chaperones facilitate the folding of proteins and are essential to regulating the quality of the cellular protein content. This ability declines during the aging process, inducing the accumulation of damaged and misfolded proteins that can lead to cell death or malfunction. Several neurodegenerative age-related disorders such as Alzheimer’s, Parkinson’s or Huntington’s disease are linked to a decline in protein quality control. Human pluripotent stem cells can replicate indefinitely while maintaining their undifferentiated state and, therefore, are immortal in culture. This capacity necessarily demands avoidance of any imbalance in the integrity of their protein content. “There is one chaperone system, the TRiC/CCT-complex that is responsible for folding about 10% of all the cellular proteins. By studying how pluripotent stem cells maintain the quality of their proteome, we found that this complex is regulated by the subunit CCT8,” says David Vilchez, senior author of the study. “Then, we discovered a way to increase the assembly and activity of the TRiC/CCT complex in somatic tissues by modulating this single subunit, CCT8. The increase resulted in prolonged lifespan and delay of age-related diseases of the model organism Caenorhabditis elegans,” he adds. “For this study we combined the results from human pluripotent stem cells and C. elegans, to have both in vitro and in vivo models, providing a more convincing approach. Our results show that expressing CCT8 as the key subunit of the complex is sufficient to boost the assembly of the whole system,” says Alireza Noormohammadi, one of the first authors of the paper. “It is very interesting that expressing this single subunit is enough to enhance protein quality and extend longevity, even in older animals,” adds Amirabbas Khodakarami, the other main author. “One of our next steps will be to test our findings in mice,” outlines David Vilchez. “We hope to make further progress in understanding aging diseases and to get closer to finding therapies against diseases like Huntington’s or Alzheimer’s. CCT8 could be a candidate to correct deficiencies in age-related diseases associated with protein dysfuntions.” Original Publication: Alireza Noormohammadi*, Amirabbas Khodakarami*, Ricardo Gutierrez-Garcia, Hyun Ju Lee, Seda Koyuncu, Tim Konig, Christina Schindler, Isabel Saez, Azra Fatima, Christoph Dieterich & David Vilchez. Somatic increase of CCT8 mimics proteostasis of human pluripotent stem cells and extends C. elegans lifespan. NATURE COMMUNICATIONS | 7:13649 | DOI: 10.1038/ncomms13649 * These authors contributed equally to this work. |

 RSS Feed
RSS Feed
